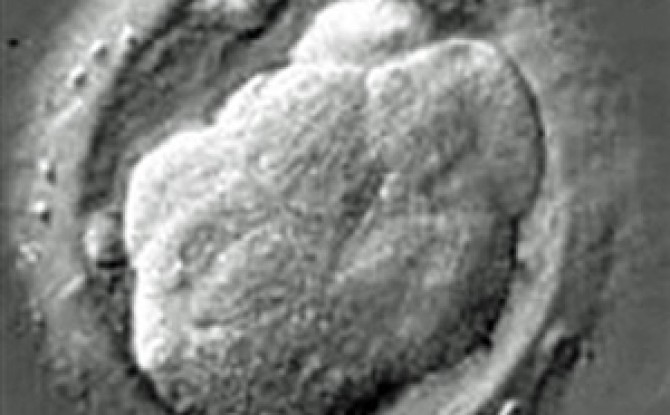
Scientists for the first time have successfully edited genes in human embryos to repair a common and serious disease-causing mutation, producing apparently healthy embryos, according to a study published on Wednesday.
The research marks a major milestone and, while a long way from clinical use, it raises the prospect that gene editing may one day protect babies from a variety of hereditary conditions.
But the achievement is also an example of genetic engineering, once feared and unthinkable, and is sure to renew ethical concerns that some might try to design babies with certain traits, like greater intelligence or athleticism.
The study, published in the journal Nature, comes just months after a national scientific committee recommended new guidelines for modifying embryos, easing blanket proscriptions but urging it be used only for dire medical problems.
“We’ve always said in the past gene editing shouldn’t be done, mostly because it couldn’t be done safely,” said Richard Hynes, a cancer researcher at the Massachusetts Institute of Technology who co-led the committee. “That’s still true, but now it looks like it’s going to be done safely soon,” he said, adding that the research is “a big breakthrough.”
“What our report said was, once the technical hurdles are cleared, then there will be societal issues that have to be considered and discussions that are going to have to happen. Now’s the time.”
Scientists at Oregon Health and Science University, with colleagues in California, China and South Korea, reported that they repaired dozens of embryos, fixing a mutation that causes a common heart condition that can lead to sudden death later in life.
If embryos with the repaired mutation were allowed to develop into babies, they would not only be disease-free but also would not transmit the disease to descendants.
The researchers averted two important safety problems: They produced embryos in which all cells — not just some — were mutation-free, and they avoided creating unwanted extra mutations.
“It feels a bit like a ‘one small step for (hu)mans, one giant leap for (hu)mankind’ moment,” Jennifer Doudna, a biochemist who helped discover the gene-editing method used, called CRISPR-Cas9, said in an email.
“I expect these results will be encouraging to those who hope to use human embryo editing for either research or eventual clinical purposes,” said Dr. Doudna, who was not involved in the study.
New York times – August 2, 201 – by Pam Belluck
Click here to read the entire article.